Chronic Back Pain Treatment
The Intracept ® is an intraosseous nerve ablation procedure used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves for the relief of chronic low back pain that has not responded to at least six months of conservative care.
The Procedure
The Intracept ®Procedure is a minimally invasive procedure that targets the basivertebral nerve for the relief of chronic vertebrogenic low back pain.
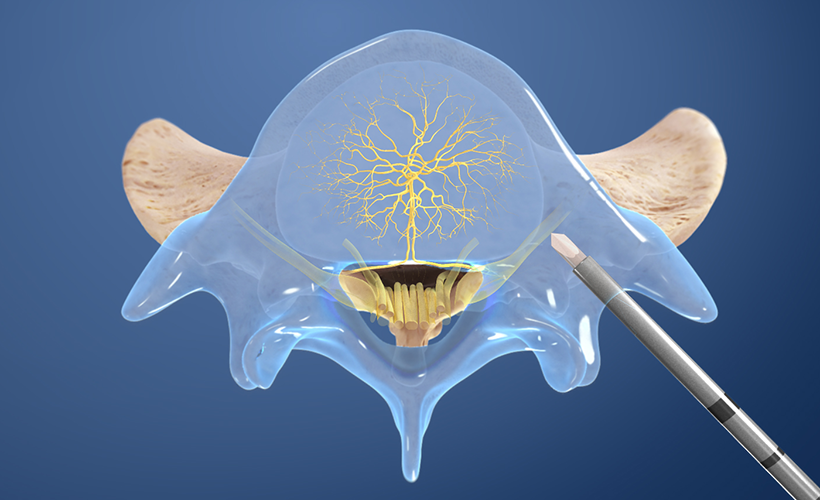
1. Access the pedicle
Under fluoroscopic guidance, the Intracept Introducer Cannula is advanced through the pedicle.
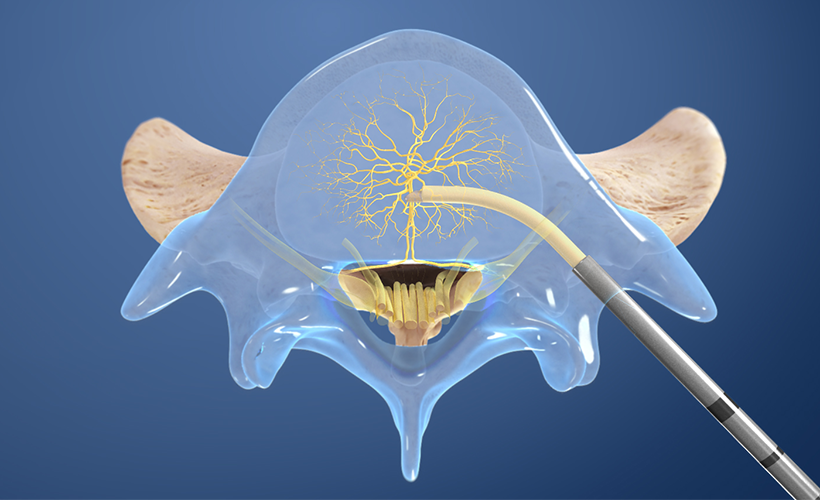
2. Create the channel
The Intracept Curved Cannula is utilized to create a channel to the trunk of the basivertebral nerve.
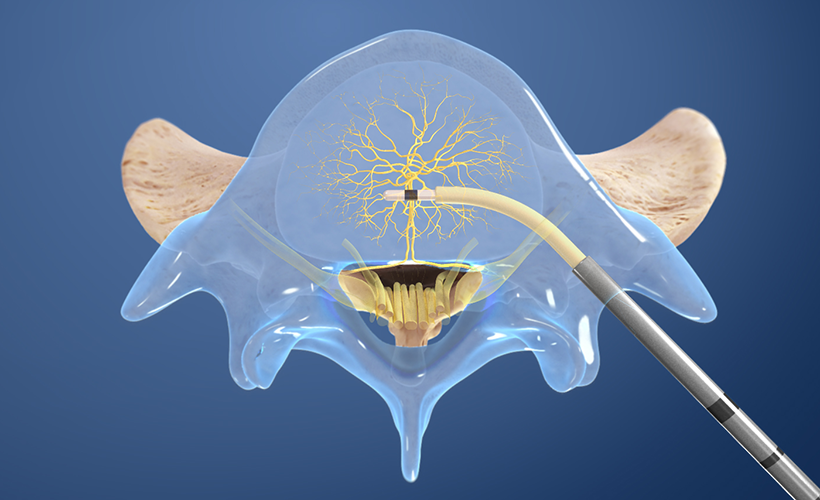
3. Place the RF Probe
The Intracept Radiofrequency Probe is inserted into the curved path and placed at the basivertebral nerve.
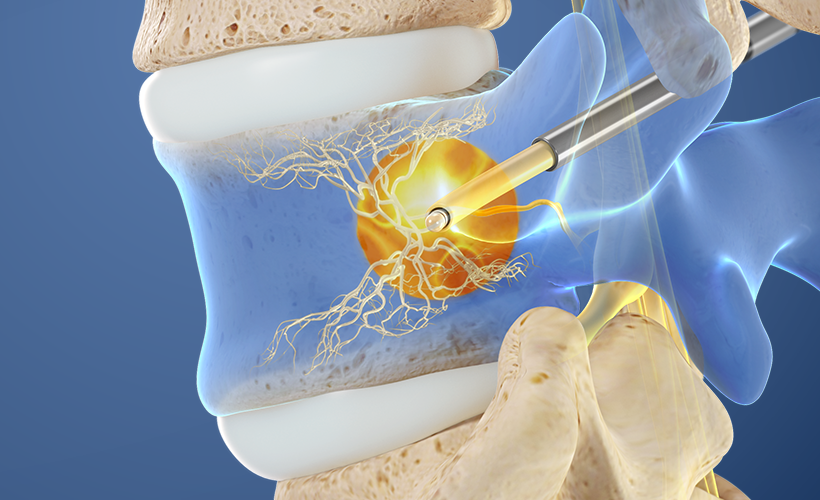
4. Ablate the BVN
The Relievant Radiofrequency Generator is utilized to ablate the basivertebral nerve.
Key Benefits
- Provides a treatment option for patients who have not responded to conservative therapy
- Minimally invasive, outpatient procedure
- Implant-free and preserves the structure of the spine
- Provides durable relief of chronic vertebrogenic low back pain
Patient Qualifications
- Chronic Low Back Pain of at least 6 months
- Not responded to at least 6 months of conservative care
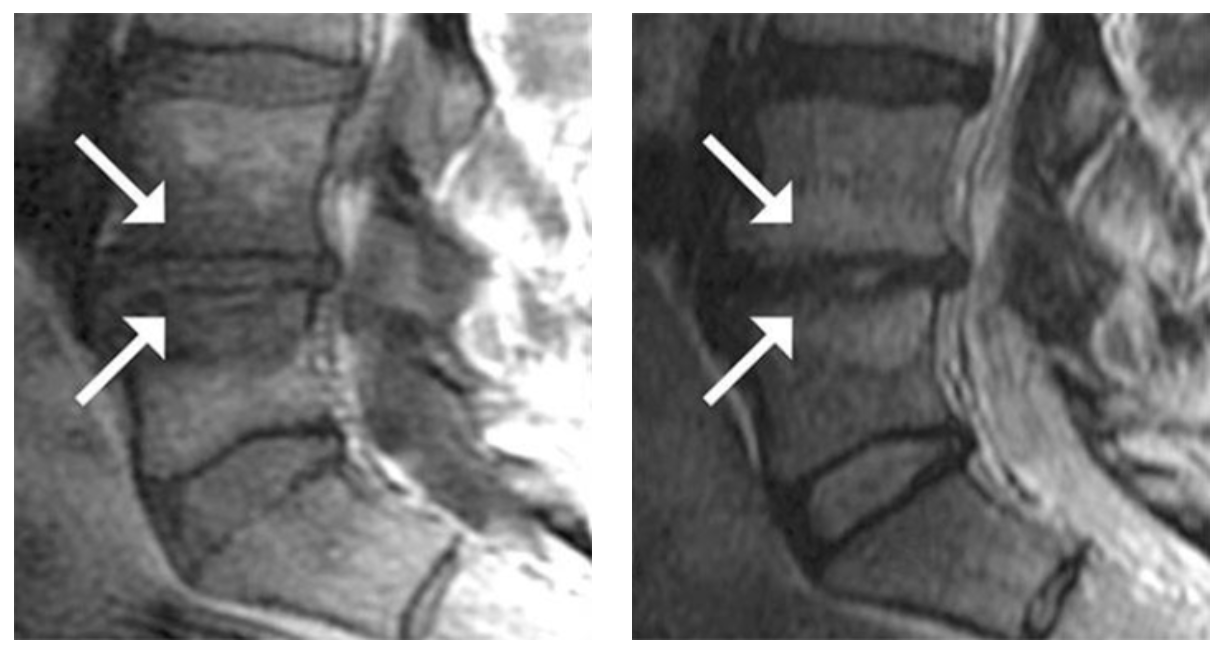
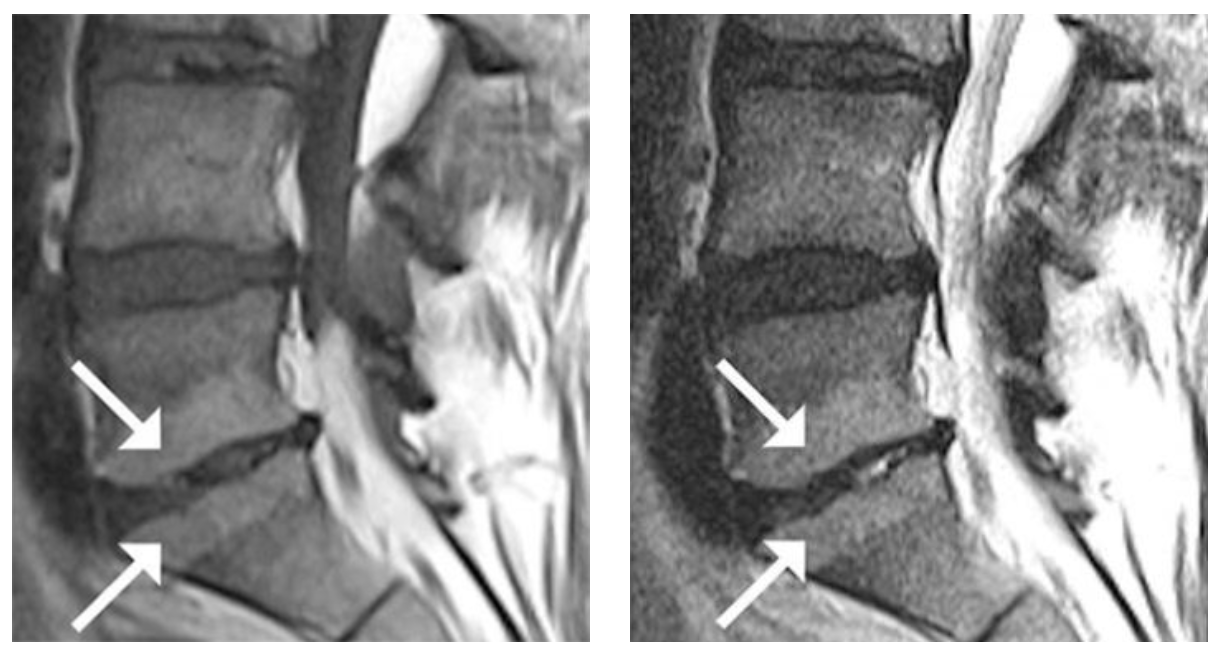
- Type 1 or Type 2 Modic changes on an MRI
Modic Type 1:
Hypointense T1W and Hyperintense T2W MR
Modic Type 2:
Hyperintense T1W and T2W MR
Indications
Chronic Vertebrogenic Low Back Pain
The Intracept Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least six months duration that has not responded to at least six months of conservative care, and is also accompanied by features consistent with Type 1 or Type 2 Modic changes on an MRI such as inflammation, edema, vertebral endplate changes, disruption and fissuring of the endplate, vascularized fibrous tissues within the adjacent marrow, hypointensive signals (Type 1 Modic change), and changes to the vertebral body marrow including replacement of normal bone marrow by fat, and hyperintensive signals (Type 2 Modic change).
Use of the Intracept Intraosseous Nerve Ablation System is contraindicated in:
- Patients with severe cardiac or pulmonary compromise.
- Patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal).
- Patients with active systemic infection or local infection in the area to be treated.
- Patients who are pregnant.
- Skeletally immature patients (generally < 18 years of age).
- Patients with implantable pulse generators (e.g., pacemakers, defibrillators) or other electronic implants.
- Situations where unintended tissue damage may result, based on the clinical assessment by the physician.
- Application with electrosurgical instruments NOT tested and specified for use with the Relievant RFG.
- As with any surgical procedure, there are risks and considerations associated with the Intracept Procedure. To review the contraindications, warnings, and precautions click here.
Patient Testimonials
Clinical Education
The Intracept Procedure is supported by two Level l randomized clinical trials. Intracept has demonstrated statistical significance compared to both standard care and sham, consistent improvement in both pain and function across both trials, and durable results beyond five years post-procedure. For more information click here.
Insurance
Relievant is committed to providing patients, physicians and facilities with resources to assist in the reimbursement process and to gain patient access for treatment with the Intracept Procedure. Relievant has a dedicated team of professionals to assist in the prior authorization of the Intracept Procedure. The prior authorization process involves obtaining advance notification from an insurance company that medical necessity and other coverage criteria have been met as set forth by the insurance company. The Relievant team works alongside the patient and physician throughout the prior authorization process in an attempt to obtain approval for the Intracept Procedure. For more information, click here.

